RNA-Guided Recombinases Enable Large-Scale Genome Engineering
RNA-Guided Recombinases Are Poised to Revolutionize Genome Editing
Subscribe to Genomely for the latest discoveries and in-depth analyses in your inbox.
Thank you to our subscribers for your continued support and passion for science!
Bananas, golden rice, featherless chickens. What do all of these have in common? They were all generated using genetic engineering, some more direct than others. However, precise and accurate genome engineering can help combat human disease, tailor crops to a warming world, and even generate glowing plants. Identifying new ways to manipulate DNA precisely will help accelerate these efforts.
In recent studies, novel genome editing approaches have been described and are poised to shake up the field. This genome engineering is achieved with RNA-guided recombinases, which offer unprecedented precision and flexibility in manipulating large DNA sequences.
The Challenge of Large-Scale Genome Editing
Biologists have dreamed of developing methods to accurately rearrange long DNA sequences within genomes for decades. The ability to insert, invert, delete, or relocate kilobase-scale DNA sequences at specific genomic locations in a single step would open up possibilities for genetic research and therapeutic applications. However, achieving this level of precision and control has been a significant challenge.
Traditional recombinase and transposase enzymes, which naturally mediate genomic rearrangements of large sequences, have been a longtime subject of study. These enzymes recognize specific DNA sequences through complex protein-DNA interactions, typically targeting sequences of 30-50 base pairs. While powerful, the complexity of these interactions has made it difficult to reprogram these enzymes to target user-specified genomic sites, limiting their utility as a flexible genome editing tool.
The CRISPR Revolution and Its Limitations
CRISPR-Cas systems marked a major breakthrough in genome editing, offering a programmable method for making precise DNA cuts. However, while CRISPR technology excels at making small edits or insertions, it has limitations when manipulating larger DNA sequences. This gap in capabilities has left researchers searching for complementary tools that could handle large-scale genomic rearrangements with the same level of programmability as CRISPR.
Enter RNA-Guided Recombinases
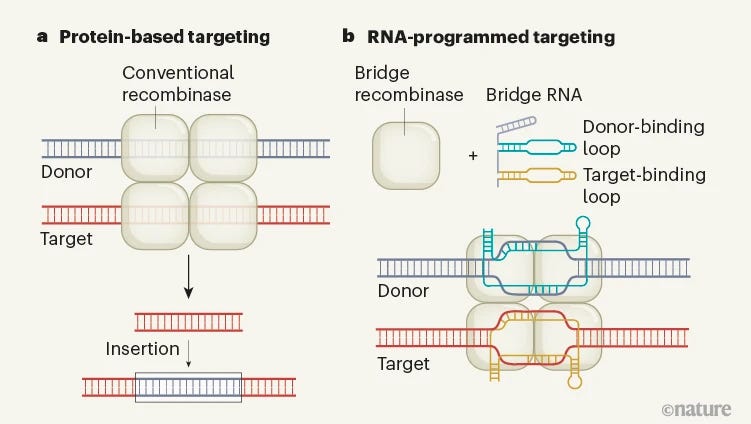
Two recent studies by Durrant et al. and Hiraizumi et al. have introduced a game-changing solution to this challenge: RNA-guided recombinase enzymes. These enzymes, derived from mobile genetic elements known as insertion sequences (ISs), use a "bridge" RNA molecule to recognize their DNA targets. This approach combines the best of both worlds – the large-scale manipulation capabilities of recombinases with the programmability of RNA-guided systems like CRISPR.
Key Features of RNA-Guided Recombinases:
Bridge RNA (bRNA) Structure: The bRNA consists of two critical regions:
A donor-binding loop (DBL) that recognizes the donor DNA molecule
A target-binding loop (TBL) that specifies the target site in the genome
Programmability: The sequences of the DBL and TBL can be independently engineered, allowing researchers to program the recombinase to perform specific operations (inversion, excision, or insertion) at user-defined genomic locations.
Versatility: These enzymes have been shown to work effectively in bacteria (specifically E. coli), with potential for application in diverse organisms.
Natural Diversity: Computational analysis has identified bRNAs in various IS110 and IS1111 elements, suggesting that a diverse array of bridge recombinases exists in nature, each with potentially unique properties that could be harnessed for genome editing.
Structural Insights and Mechanism
Hiraizumi et al. have provided valuable structural information about these RNA-guided recombinases through high-resolution imaging of IS621 complexes at various stages of DNA rearrangement. These structures reveal:
The intricate molecular interactions that enable pairing between the bRNA and its DNA targets
The architecture of the active site responsible for DNA cleavage
The role of specific amino acid residues in destabilizing DNA duplexes to facilitate bRNA-mediated recognition
This structural information not only elucidates the mechanism of action but also provides a blueprint for future engineering efforts to enhance the efficiency and specificity of these enzymes.
Potential Applications and Implications
The discovery of RNA-guided recombinases opens up a wealth of possibilities for genetic research and biotechnology:
Precise Genomic Integration: These enzymes could allow for the accurate insertion of large DNA sequences, such as entire genes or regulatory elements, at specific locations in the genome.
Gene Therapy: The ability to precisely insert large DNA sequences could enhance gene therapy approaches, potentially allowing for the correction of complex genetic disorders that require the insertion of full-length genes.
Synthetic Biology: RNA-guided recombinases could facilitate the construction of complex synthetic genetic circuits by enabling the precise arrangement of large DNA modules within genomes.
Evolutionary Studies: These tools may provide new ways to study genome evolution and the role of large-scale genomic rearrangements in adaptation and speciation.
Agricultural Applications: In crop improvement, these enzymes could allow for more sophisticated genetic modifications, such as the insertion of entire biosynthetic pathways to enhance nutritional content or stress resistance.
Challenges and Future Directions
While the discovery of RNA-guided recombinases represents a significant advancement, several challenges and areas for future research remain:
Expanding Host Range: Current studies have focused on E. coli. Further research is needed to adapt these systems for use in other organisms, particularly mammalian cells.
Improving Specificity: The relatively short binding loops of current bRNAs may lead to off-target effects in large genomes. Strategies to extend these loops or discover recombinases with naturally longer binding regions will be crucial for enhancing specificity.
Optimizing Efficiency: As with any new technology, efforts to improve the efficiency of DNA rearrangement events will be important for practical applications.
Understanding Host Interactions: Further investigation into how these recombinases interact with host factors could provide insights for optimizing their function in different cellular contexts.
Exploring Natural Diversity: The vast array of mobile genetic elements in nature may harbor other RNA-programmable functions waiting to be discovered and harnessed for biotechnology.
The discovery of RNA-guided recombinases marks a significant milestone in genome editing. By combining the large-scale manipulation capabilities of recombinases with the programmability of RNA-guided systems, these enzymes offer a powerful new tool for genetic engineering. As research in this area progresses, we can expect to see rapid developments in the basic understanding of these systems and their application to various biological and biotechnological challenges.
References:
https://www.nature.com/articles/d41586-024-01461-2
https://www.nature.com/articles/s41586-024-07552-4
https://www.nature.com/articles/s41586-024-07570-2